Introduction: Infections lead to substantial morbidity during treatment of acute lymphoblastic leukemia (ALL). During ALL treatment, the adaptive immune system gets severely affected, leading to, amongst other things, declining serum immunoglobulin (IgG) levels. Theoretically, one may partially overcome the infection risk by supplementing the low IgG levels with intravenous immunoglobulins (IVIG). The aim of this trial was to investigate whether IVIG prophylaxis in pediatric patients with ALL prevents admissions for fever.
Patients and Methods: This randomized controlled trial was a subtrial of the national Dutch multicenter ALL study (DCOG ALL-11). Patients aged 1-19 years with medium risk (MR) ALL were randomized into either IVIG prophylaxis (0.7 g/kg IVIG three-weekly, starting day 22 after diagnosis) or control group (standard of care). The primary endpoint was number of admissions for fever. Secondary endpoints were antibiotic treatments, blood culture results, adaptation of chemotherapy, ICU admissions, relapse, disease-free survival (DFS), and overall survival (OS). To account for possible correlations between episodes within the same patient, generalized estimating equation models were fitted for admissions for fever, therapeutic antibiotics, chemotherapy adaptations and ICU admissions, including age in the models.
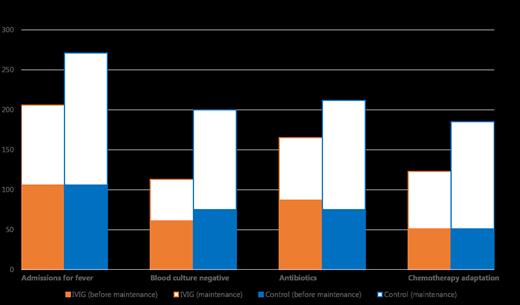
Results: Between October 2012 until March 2019, 91 (51%) patients were randomly assigned to IVIG prophylaxis and 86 (49%) to the control arm. In the IVIG prophylaxis group there were 206 admissions for fever versus 271 in the control group (p=0.026, Figure). IVIG prophylaxis was associated with significantly less admissions for fever with negative blood cultures compared to the control group (N=113 versus 200, for IVIG prophylaxis versus control, respectively, p=0.015, Figure), and a significant decrease of chemotherapy adaptations (N=123 versus 185, for IVIG prophylaxis versus control, respectively, p=0.021, Figure).
As post-hoc analysis we studied in which treatment phase IVIG prophylaxis was most relevant. Specifically during maintenance treatment of ALL, IVIG prophylaxis was associated with significantly less admissions for fever (N=100 versus 165, for IVIG prophylaxis versus control, respectively, p=0.002, Figure), with negative blood cultures (N=52 versus 125, for IVIG prophylaxis versus control, respectively, p=0.015, Figure), resulting in a significant decrease of chemotherapy adaptations (N=72 versus 134, for IVIG prophylaxis versus control, respectively, p=0.002, Figure) and antibiotic treatments (N=78 versus N=137, for IVIG prophylaxis versus control, respectively, p=0.005, Figure). There was no significant impact on relapse, DFS or OS.
Conclusion: In pediatric patients with MR ALL, IVIG prophylaxis is associated with significantly less admissions for fever with negative blood cultures, during maintenance treatment, resulting in a decreased number of chemotherapy adaptations and antibiotic treatments. Future studies should aim at identifying a subgroup of patients that benefit most from IVIG prophylaxis.
Figure: Admissions for fever; with negative blood cultures, courses of antibiotics, or chemotherapy adaptation for IVIG prophylaxis and control group
*: p<0.05 during entire ALL treatment; #: p<0.05, ##: p<0.01 during maintenance phase of ALL treatment separately.
Disclosures
Van Der Sluis:Servier: Consultancy. Zwaan:ITCC Hem Malignancies Committee: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Incyte: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy; Incyte: Consultancy; Gilead: Consultancy; Novartis: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Daiichi Sankyo: Other: Institutional support for clinical trials; Gilead: Other: Institutional support for clinical trials; Kura: Other: Institutional support for clinical trials; Jazz: Other: Institutional support for clinical trials; Chair MREC Utrecht: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Chair Dutch MREC Society: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Takeda: Other: Institutional support for clinical trials; Abbvie: Other: Institutional support for clinical trials; Pfizer: Other: Institutional support for clinical trials. van Tilburg:Alexion: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Pieters:Clinigen: Consultancy; Servier: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal